Sativex and Multiple Sclerosis
It has been recognized by some medical clinicians and scientific researchers studying cannabinoids that the cannabis plant may hold compounds useful in helping to treat multiple sclerosis (MS) and neuromuscular spasticity. This area of research has been ongoing rather actively for over 20 years, and perhaps even for hundreds or thousands of years in general throughout the ages. Cannabinoids have been long known to have effects on reducing or mitigating neuropathic pain – this is pain caused by and originating in the nerves themselves. Multiple Sclerosis is such a disease where the neural sheath and protective covering of neurons within the nervous system are suffering degradation and cellular damage, which comes with a wide variety of unwanted symptoms, partly treated by existing medications.
The early warning signs and symptoms of MS are vision problems, tingling and numbness, balance issues, bladder problems, cognitive problems, pains and spasms, and weakness and fatigue. Not all patients present with all of these symptoms as the disease expression can be variable. It is typically diagnosed based on the presenting signs and symptoms and can be confirmed by lab blood tests and medical imaging. Often difficult to diagnose at first as it is similar to other diseases, and often patients see the doctor several times before the diagnosis is made.
According to most research studies and current guidelines, patients with MS should seek and receive regular medical care including drugs and prescriptions. This disease is often well managed with current therapies. However, some areas of improvement are possible and some cases are refractory to current medications. This is where the drug Sativex has been clinically recognized to be useful as an adjunct to current therapy in this disease. Note that cannabis and Sativex should be used in addition to regular medication and care when treating this disease, not in place of it. Traditional medication can be quite effective in treatment, but the use of Sativex may positively augment care and well-being for these patients. Also, Sativex can be beneficial in refractory cases where other medications are not effective.
Sativex has been investigated for over 10 years and has proven efficacy in helping to treat the symptoms of MS. It is manufactured by GW Pharmaceuticals in Great Britain, and has been in use in other countries around the globe, but banned in the US because it contains THC still federally illegal. It is administered as a sublingual tincture or spray, is manufactured solely from the cannabis plant, and has approximately equal parts of THC and CBD, as well as other cannabinoids. Because of the presence of an equal amount of CBD, the THC psycho-activity is greatly diminished and this allows for fewer unwanted side effects, with less mental and cognitive involvement. It is interesting to note that this product is not available at traditional pharmacies in our country, but dispensaries in Maryland have similar products that can be substituted for Sativex if selected carefully. Several of my own patients have demonstrated success with some of these products, which can be used until Sativex becomes available in this country. Due to the nature of the disease, and also due to the wide variety of different strains, terpenes, and types of cannabis and extracts, it may take several weeks of experimentation to determine if it will be beneficial. Patient response and subjective interpretation of benefits can give altered or inaccurate results of treatment and is highly variable. Nonetheless, Sativex has been demonstrated clinically in a large sample of patients over years of research to be useful in MS – and positive anecdotal stories are also quite common.
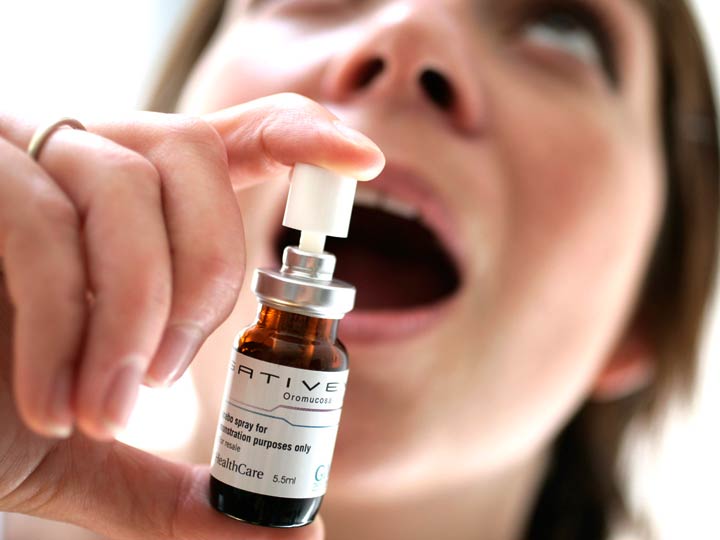
Taking a look at Sativex, let’s explore some more details and information concerning dosages, methods of administration, and side effects, as well as expected beneficial therapeutic effects. Sativex has 2.7 mg delta-9-tetrahydrocannabinol (THC) and 2.5 mg of cannabidiol (CBD) from cannabis Sativa L plants as well as a small amount of ethanol used in processing. According to the manufacturer, Sativex is indicated “for treatment in symptom improvement in patients with moderate to severe spasticity due to multiple sclerosis who have not responded adequately to other anti-spasmodic medication and who demonstrate clinically significant improvement in spasticity related symptoms during an initial trial of therapy”.
As an oromucosal spray, it is applied to the tissues inside your mouth (the oral mucosa) which include the insides of your cheeks, your palate, your gums, and your tongue, also underneath your tongue (sub-lingual) – and some can also be swallowed but the effect will be delayed. The normal onset of the spray direct contact with oral mucosa is about 20 minutes. If swallowed and ingested via the GI tract the onset will take about 1 to 1-1/12 hours. It can be used in adults only over the age of 18 under the supervision of an experienced caregiver, in conjunction with regular therapy. A titration period of a few weeks is recommended, and during this time the dosage is monitored and corrected as needed, but the average dose is 8 sprays a day. Patients should report a 20% reduction in spasticity after using Sativex for one month, or else it was not recommended to be continued as part of their care. Side effects include dizziness, fainting, but no significant alteration in blood pressure or cardiac function.
Its mechanism of action is on the CB1 and CB2 cannabinoid receptors which are mainly located on the nerve terminal ends on the pre-synaptic neurons. This will play a role in retrograde regulation of synaptic function and subsequent alteration in neurotransmitter release of chemicals similar to glutamate or serotonin, etc… This directly can affect pain signaling and reduce neuronal transmission of pain. In one study of 572 patients after 4 weeks of therapy with Sativex, 241 achieved a 20% reduction in spasticity – so it was fairly effective in about half of the patients in this one particular trial.
As with any therapy using cannabinoids, careful medical supervision is required and patients and doctors must be able to titrate and maintain the proper dose while mitigating any unwanted side effects. The use in elderly patients requires special care and moderation of therapy to minimize adverse effects. I would surmise that those receiving therapy with Sativex could also expect to mitigate any excessive psychoactive effects thru the use of 15 mg CBD capsules ingested orally or vaporized if required. Safety is similar to other cannabis products, with no lethal dose, and any adverse side effects quite manageable. Patients with MS will now have this option available for treatment with tinctures/sprays similar to Sativex, and perhaps soon the US will allow the patented product to be available as well. Patients who are using this drug under proper supervision, along with traditional therapy, can be the ones to decide if it is helpful for them. If MS patients feel it is helping them with their disease and quality of life, then this is what patient-based medicine is all about.